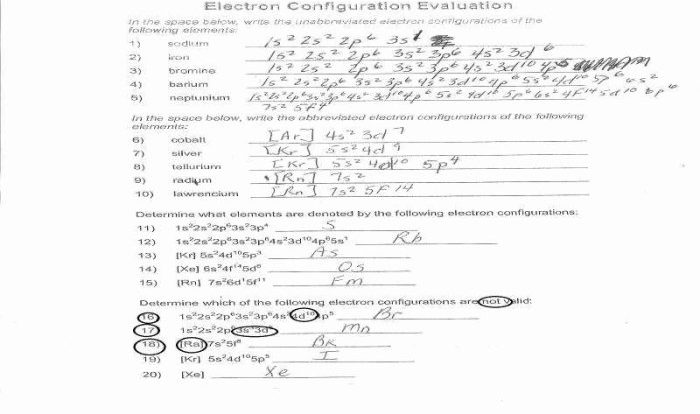
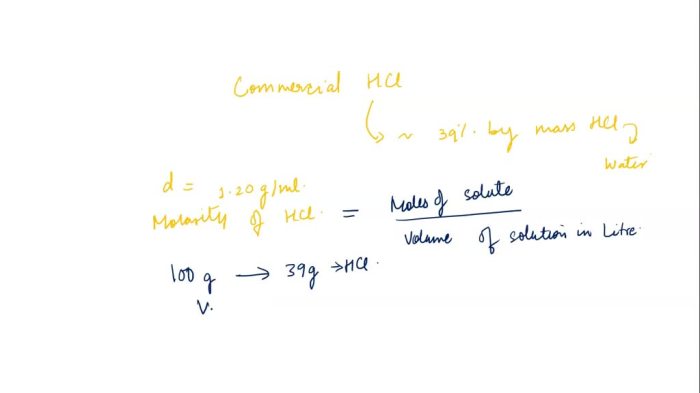
Commercial grade hcl solutions are typically 39.0 – Commercial grade HCl solutions, typically characterized by a concentration of 39.0%, play a significant role in various industries and laboratory applications. Their unique properties and wide-ranging uses make them an indispensable chemical reagent.
This article delves into the concentration, properties, applications, safety considerations, and comparison of commercial grade HCl solutions with other commonly used acids, providing a comprehensive overview of this essential chemical.
Concentration and Strength: Commercial Grade Hcl Solutions Are Typically 39.0
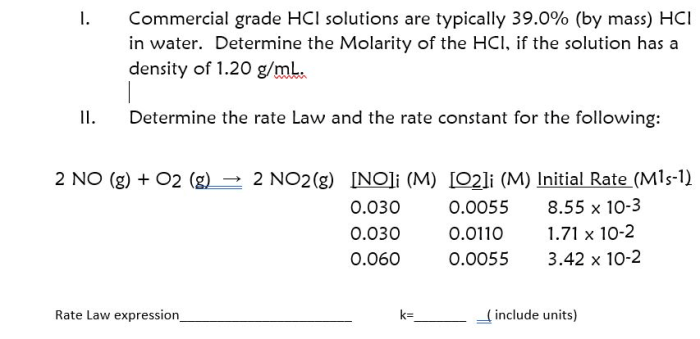
Commercial-grade HCl solutions typically have a concentration of 39.0%, which is a highly concentrated form. This concentration is significant for various applications as it provides a balance between effectiveness and safety. It is strong enough to be effective in a wide range of applications while minimizing the potential for excessive corrosion or other hazards.
Industries and applications where this concentration is commonly used include:
- Metalworking and electroplating
- Chemical manufacturing
- Food and beverage processing
- Water treatment
- Laboratory analysis
Properties and Characteristics
Commercial-grade HCl solutions have several distinct physical and chemical properties:
- Density:Around 1.19 g/mL at 20°C
- Viscosity:Slightly higher than water
- Corrosive nature:Highly corrosive, can damage metals, skin, and other materials
Handling and storage requirements for these solutions include:
- Store in a cool, well-ventilated area away from incompatible materials
- Use appropriate personal protective equipment (PPE) when handling
- Store in tightly sealed containers to prevent evaporation and contamination
The stability and shelf life of commercial-grade HCl solutions depend on factors such as storage conditions, temperature, and exposure to air. Generally, they have a shelf life of several months to a year if stored properly.
Applications and Uses
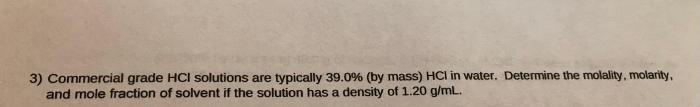
Commercial-grade HCl solutions have numerous industrial and laboratory applications:
- Metalworking and electroplating:As a pickling agent to remove oxides and impurities from metal surfaces
- Chemical manufacturing:As a reactant or catalyst in various chemical processes
- Food and beverage processing:As an acidulant or pH adjuster in food and beverage products
- Water treatment:For pH adjustment and disinfection
- Laboratory analysis:As a reagent in titrations, acid-base reactions, and other analytical procedures
Advantages of using HCl solutions include their effectiveness, low cost, and wide availability. However, their corrosive nature and potential for hazardous reactions require careful handling and storage.
Safety Considerations
Handling and using commercial-grade HCl solutions require proper safety considerations:
- Potential hazards:Corrosive to skin, eyes, and respiratory tract; can cause burns and irritation
- Personal protective equipment (PPE):Wear gloves, goggles, protective clothing, and a respirator when handling
- Safety protocols:Handle in a well-ventilated area; avoid contact with skin and eyes; neutralize spills with a base
- Emergency procedures:In case of contact, flush affected areas with water and seek medical attention immediately
It is crucial to follow proper safety protocols and training when working with commercial-grade HCl solutions to minimize risks and ensure a safe working environment.
Comparison to Other Acids
Commercial-grade HCl solutions can be compared to other commonly used acids such as sulfuric acid (H2SO4) and nitric acid (HNO3):
| Property | HCl | H2SO4 | HNO3 |
|---|---|---|---|
| Concentration | 39.0% | 98% | 68% |
| Strength | Strong | Strong | Strong |
| Corrosiveness | Highly corrosive | Highly corrosive | Highly corrosive |
| Applications | Metalworking, chemical manufacturing, food processing | Fertilizer production, metal refining, batteries | Rocket propellants, explosives, fertilizers |
The choice between HCl and other acids for specific applications depends on factors such as the desired reaction, pH requirements, and safety considerations.
FAQ Section
What is the typical concentration of commercial grade HCl solutions?
39.0%
What are the major applications of commercial grade HCl solutions?
Industrial processes, laboratory analysis, metalworking, and acid cleaning
What safety precautions should be taken when handling commercial grade HCl solutions?
Wear appropriate personal protective equipment, handle in well-ventilated areas, and follow proper disposal procedures